Description
COMPOSITION
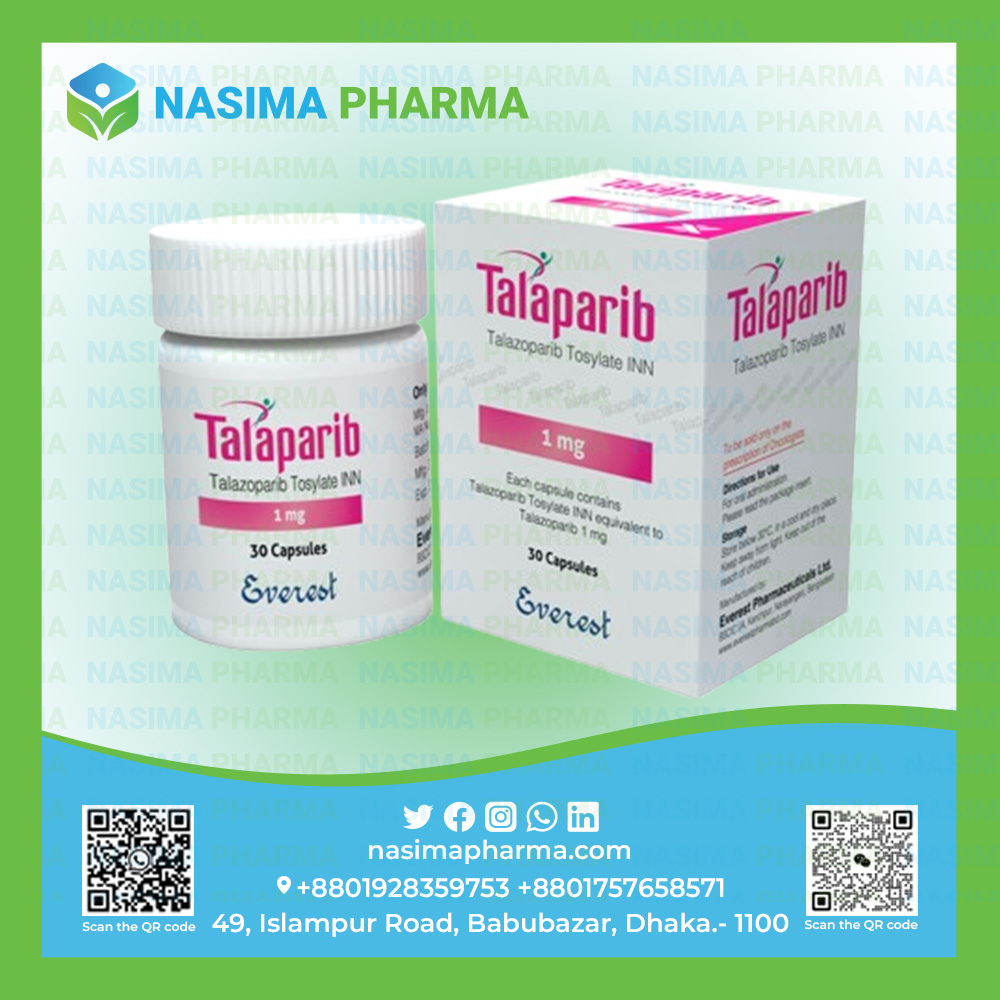
TALAPARIB capsule: Each capsule contains Talazoparib Tosylate INN equivalent to Talazoparib 1 mg.
PHARMACOLOGY
Mechanism of Action
Talazoparib is an inhibitor of poly (ADP-ribose) polymerase (PARP) enzymes, including PARP1 and PARP2, which play a role in DNA repair. In vitro studies with cancer cell lines that harbored defects in DNA repair genes, including BRCA1 and BRCA2, have shown that Talazoparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes resulting in DNA damage, decreased cell proliferation, and apoptosis. Talazoparib anti-tumor activity was observed in patient-derived xenograft breast cancer models bearing mutated BRCA 1 or mutated BRCA2 or wild type BRCA 1 and BRCA2.
PHARMACODYNAMICS
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of Talazoparib have not been fully characterized.
Cardiac Electrophysiology
At a dose of 1 mg (the recommended dosage for treatment of breast cancer), Talazoparib had no large QTc prolongation (i.e., >20 ms).
PHARMACOKINETICS
After administration of Talazoparib 1 mg orally once daily as a single agent (the recommended dosage for breast cancer), the mean [% coefficient of variation (CV%)] AUG and maximum observed plasma concentration (Cmax) of Talazoparib at steady-state was 208 (37%) ng.hr/mL and 16.4 (32%) ng/mL, respectively. The mean (CV%) steady-state Ctrough was 3.53 (61 %) ng/ml. After administration of Talazoparib 0.5 mg orally once daily (the recommended dosage for prostate cancer) in combination with Enzalutamide, the mean (CV%) steady-state Ctrough ranged from 3.29 to 3.68 ng/ml (45% to 48%). The pharmacokinetics (PK) of Talazoparib is linear from 0.025 mg to 2 mg (2 times the recommended dose for breast cancer). The median accumulation ratio of Talazoparib following 1 mg orally once daily is 2.3 to 5.2. Talazoparib plasma concentrations reached steady-state within 2 to 3 weeks when administered as a single agent and within 9 weeks when coadministered with Enzalutamide.
Absorption
The median time to Cmax (Tmax) was generally between 1 to 2 hours after dosing.
Distribution
The mean apparent volume of distribution of Talazoparib is 420 L. In vitro, protein binding of Talazoparib is 74% and is independent of Talazoparib concentration.
Elimination
The mean terminal plasma half-life (±standard deviation) is 90 (±58) hours and the mean apparent oral clearance (inter-subject variability) is 6.45 L/h (31 %).
Metabolism
Talazoparib undergoes minimal hepatic metabolism. The identified metabolic pathways include mono-oxidation, dehydrogenation, cysteine conjugation of monodesfluoro-talazoparib, and glucuronide conjugation.
Excretion
Excretion of Talazoparib in urine was the major route of elimination. Approximately 68.7% (54.6% unchanged) of the total administered radiolabeled dose of Talazoparib was recovered in urine, and 19.7% (13.6% unchanged) was recovered in feces.


Reviews
There are no reviews yet.