Description
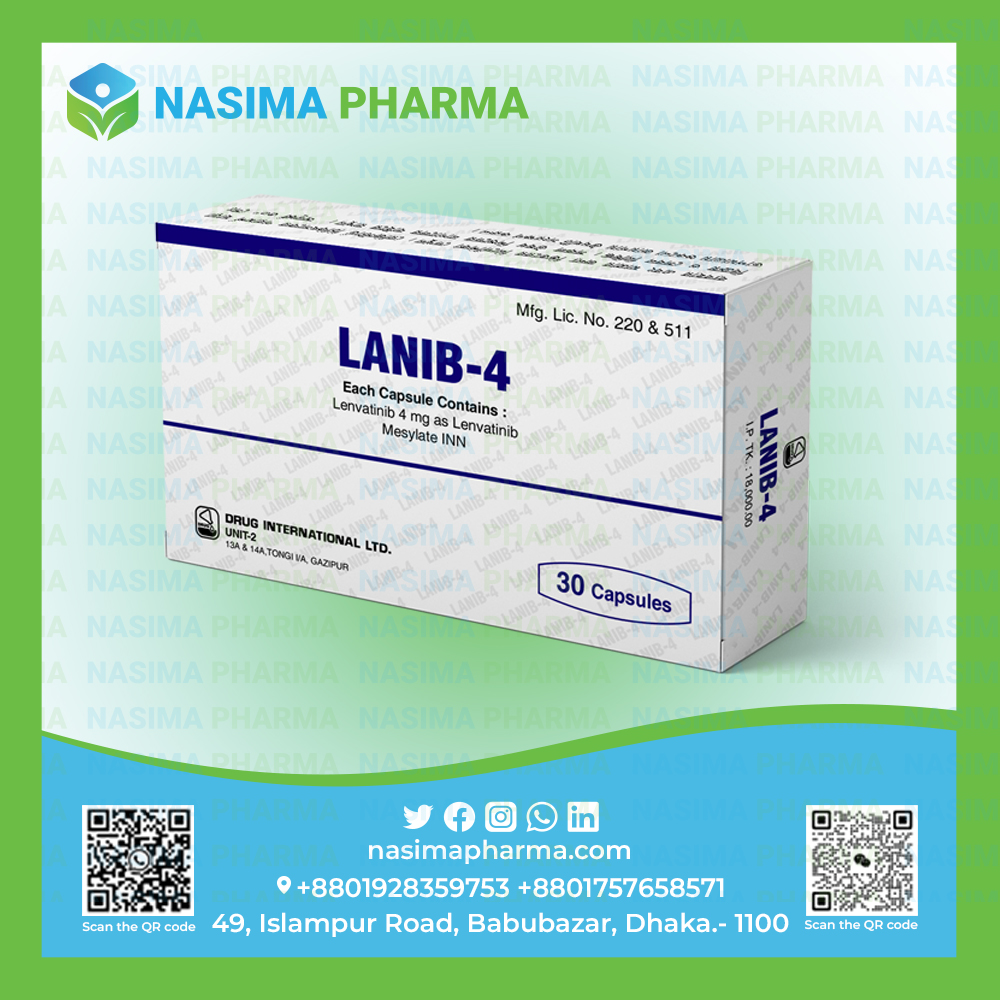
Lanib-4
Lenvatinib Mesylate INN
Composition: Each capsule contains Lenvatinib 4 mg as Lenvatinib Mesylate INN.
Indications: Differentiated Thyroid Cancer: Lenvatinib is indicated for the treatment of patients with locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer (DTC). Renal Cell Carcinoma: Lenvatinib is indicated in combination with Everolimus for the treatment of patients with advanced renal cell carcinoma (RCC) following one prior anti-angiogenic therapy. Hepatocellular Carcinoma: Lenvatinib is indicated for the first-line treatment of patients with unresectable hepatocellular carcinoma (HCC). Endometrial Carcinoma: Lenvatinib, in combination with Pembrolizumab, is indicated for the treatment of patients with advanced endometrial carcinoma.
Dosage and Administration: Recommended Dosage for Differentiated Thyroid Cancer (DTC): The recommended dosage of Lenvatinib is 24 mg orally once daily until disease progression or until unacceptable toxicity. Recommended Dosage for Renal Cell Carcinoma (RCC): The recommended dosage of Lenvatinib is 18 mg in combination with 5 mg Everolimus orally once daily until disease progression or until unacceptable toxicity. Recommended Dosage for Hepatocellular Carcinoma (HCC): The recommended dosage of Lenvatinib is based on actual body weight. Or, as directed by the registered physician.
Use in Pregnancy and Lactation: Based on the mechanism of action, Lenvatinib can cause embryo-fetal harm when administered to a pregnant female. Pregnant women should be advised of the potential risk to a fetus. Females of reproductive potential should be advised to use effective contraception during treatment with Lenvatinib and for at least 30 days after the last dose.



Reviews
There are no reviews yet.